Techniques – Monoclonal Antibodies Profiling (mAbs)
Introduction:
In order to assess the bioactivity and safety of recombinant monoclonal antibodies (mAbs) as biological drugs, it is important to fully characterise them. Not only is this information crucial for the assessment of the suitability of a potential biopharmaceutical product, it is also important for the optimisation and QA/QC of the manufacturing process, storage conditions and life-time assessment of the drug.
Challenge:
The mAb Rituximab is a biological drug used in the treatment of arthritis as well as a number of other inflammatory and autoimmune diseases. Unlike the chemical drugs in common use for decades biological drugs are not robust and can fragment if not stored and used properly. These drugs are often administered by slow injection as saline infusions over a period of several hours. The manufacturers supply these drugs in standard size vials in the £800 ($1000) price bracket. They are then formulated in the hospital in new manufacturing centres for delivery to the patient. In the UK the current National Health Service (NHS) protocol dictates that once an infusion is reconstituted it must be administered within 24 hours or discarded. Any remaining drug left in the saline infusion and the vial is also discarded after this time. This leads to high levels of drug wastage and contributes to high treatment costs. The ability to test whether the drug is still safe to administer following reconstitution, after storage as stock, would prevent wastage and lead to significant cost savings for the NHS. This requirement to prevent wastage will become increasingly important as worldwide healthcare organisations increasingly move to stratified biological therapies delivered to individual patients based on their genetic profiles.
Rationale:
Analytical departments within the healthcare industry have not seen the investment in equipment and personnel required to prevent such wastage. The use and formulation of biological drugs is not a mainstream pharmaceutical process yet, as many of the pharmacists will be more trained in delivering chemical formulations. In routine patient care diagnostic testing has been outsourced to many third party analytical companies while many NHS hospitals have excellent in-house analytical facilities. However neither of these routes provide the on-the-spot biochemical grade analytical tools needed for the increasing use of biological drugs and the need to monitor e.g. mAbs from delivery to the hospital to injection point at the bedside, This workflow includes the stability in storage of both stock and saline infusion bag along with the standardisation of how much intact drug remains efficacious to the patient. This set of standards, or metrics, are now being developed by NHS quality control laboratories, with active input from deltaDOT staff, using the NHS’s own HPCE-512 systems. Traditionally protein analysis has been performed on standard wet electrophoresis gels, which is time consuming and not very repeatable. Other techniques, such as HPLC and Mass Spectrometry are expensive, hard to use and need expert users. They are not very practical for this sort of highly precise analytical requirement and as the complexity of analysis increases, they become less and less attractive. Traditional single point detection capillary electrophoresis has suffered in repeatability and resolution measurements and has not been able to address this emerging market need.
deltaDOT Solution:
The deltaDOT HPCE-512 system uses multipoint detection over a 512-pixel photodiode array. Coupled with their proprietary signal processing software packages this system delivers higher quality data consistently on an easy to use platform. The system needs no additional visualisation chemicals to detect, analyse and acquire the data in a format that requires no additional software or training to deliver stability and efficacy data on the biological drug.
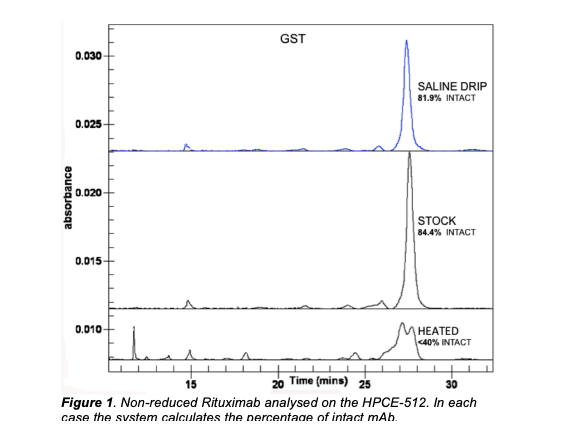
Label Free Intrinsic Imaging (LFII® ) analysis was carried out on the monoclonal antibodies Infliximab and Rituximab for the assessment of stability, fragmentation and charge heterogeneity. This work was performed as part of a study for the UK’s National Health Service (NHS). Quantifiable differences were observed in the samples before and after heat-stress. Excellent repeatability was also obtained with a peak area Relative Standard Deviation (RSD) of 2.88% and a peak time RSD of 2.28%. mAb samples were diluted to a concentration of 0.5mg/ml in Capillary Gel Electrophoresis (CGE) sample buffer and run under reducing and non-reducing conditions. The reduced samples were treated with β-mercaptoethanol and heated at 70°C for 10 minutes. The non-reduced samples were heated at 70°C for 10 minutes with no reductant.
Commercial Applications:
The high quality data produced by deltaDOT’s HPCE can be successfully used as an alternative existing mAb analysis techniques in many areas, as follows:
- Drug quality throughout the production process.
- Batch release to the consumer.
- Stock quality control on receipt of drugs from manufacturer.
- Stock stability in fridge storage.
- Drug efficacy on formulation
- Drug stability in formulation and administration to patient